Synthesis and Electrochemical Characterization of LiVMoO6, Obtained By Melt Quenching Method for AllSolid-State Lithium Batteries
Abstract
Crystalline LiVMoO6 with bannerette structure was synthesized by a novel melt quenching method followed by heat treatment in order to increase the degree of crystallinity of the material. XRD, Raman and SEM investigations were performed to examine the phase formation, local structure and morphology of the obtained product. The electrochemical properties of all-solid-state-cell using LiVMoO6 as an active material were examined. Discharge-charge measurements for 10 cycles were performed in the potential range from 1.8 to 3.7 V under a current density of 0.1 mA cm-2 at room temperature. The assembled all-solid-state Li-In/80Li2S.20P2S5 glass-ceramic/LiVMoO6 battery showed poor electrochemical performance. The charge and discharge capacities began to fade immediately after the first cycle, dropping at about 83% by the 10th discharge.
Keywords: Melt quenching technique; Cathode; All‐solid‐state battery
Introduction
The introduction by Sony in 1990 of the world's first commercially successful rechargeable lithium battery represented a revolution in the power source industry [1]. The performance of rechargeable Li-ion batteries depends on the properties of cathodes, anodes and electrolytes. That is why the search of newer materials, has opened a new era in the solid- state materials research. Many review papers and books have been published recent time [2-5], reveling the importance of rechargeable Li-ion batteries in the modern technology area. The main challenges of Li-ion technology are related with the enhancement of the energy efficiency and safety and to reduce the cost of their production [6]. Traditionally electrode active materials for Li-ion batteries are based on transition metals oxide compounds because of their open crystalline structures and high redox potential [3]. Among them the brannerite-type LiVMoO6 has attracted special attention both as cathode and anode material because of its open structure and interesting characteristic from a stand point of the variety of oxidation state and has been extensively studied as electrode active material in lithium batteries using conventional liquid electrolyte [7-11].Recently we have reported for the first time data concerning the electrochemical performance of LiVMoO6 obtained by soft- mechanochemical syhthesis as cathode active material for the all-solid-state batteries [12]. It was found that although LiVMoO6 delivered a low specific capacity it showed a stable cycling behavior and can be further investigated as an active material for the preparation of all-solid-state batteries. Herein we present the results of synthesis, structural and electrochemical characterization of LiVMoO6, obtained by melt quenching method and its electrochemical behavior as composite positive electrode in all-solid-state batteries with 80Li2S.20P2S5 glass ceramic as a solid electrolyte.
Materials and methods
LiVMoO6 was obtained by melt quenching method. The sample was prepared using reagent grade Li2CO3, V2O5 and MoO3. The homogenized batch of the starting compounds was melted for 20 min at 800 °C in alumina crucibles in air atmosphere. Crystalline material was obtained by pouring and pressing of the melt between two stainless steel plates. The quenched sample was annealed at 500 °C in air for 5 hours in order to increase the degree of crystallinity of the material and to improve its electrochemical performance. The phase formation was checked by XRD (CuKa, Ultima IV; Rigaku Corp.). The room temperature Raman measurement of LiVMoO6 was performed in the range 200‐1200cm"1 on a mcirco-Raman system from Jobin‐Yvon Horiba (LABRAM HR-800) spectrometer with green laser (wavelength: 532nm). The morphology and microstructure of LiVMoO6 were investigated by a scanning electron microscope (JEOL, JSM- 5300). Electrochemical performance of LiVMoO6 obtained was tested by assembling of all‐solid‐state cells employing LiVMoO6 as an active material. 80Li2S.20P2S5 (mol%) glass‐ceramic and Li-In alloy were respectively used as a solid electrolyte and a counter/reference electrode. The details of the construction of the all‐solid-state test cell were described in ref [12]. The prepared all‐solid‐state cell was discharged and charged in the potential range from 1.8 to 0.1 mA cm‐ under a current density of 0.1 mA cm‐ at room temperature in Ar atmosphere using a charge‐discharge measuring device (BTS‐2004; Nagano). Electrochemical impedance measurements of the prepared test cells before and after discharge‐charge measurements were performed using a charge‐discharge measuring device (SI1260 Solartron) in the frequency range from 100mHz to 1MHz.
Results and Discussion
Synthesis and structural characterization of LiVMoO6phase
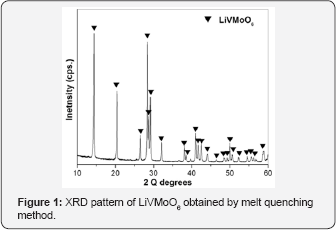
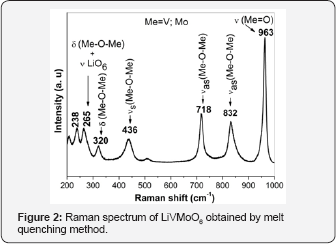
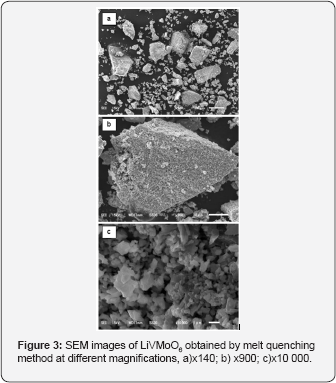
The XRD pattern of prepared LiVMoO6 is presentin Figure 1. The diffraction pattern of the obtained LiVMoO6 was indexed using “Index” software, assuming a C2/m symmetry that corresponds to the monoclinic citing of the brannerite structure and shows no traces of impurity phases [13]. The diffraction peaks are intense and symmetrical evidencing the formation of a well crystallized LiVMoO6 product. Additional information about phase formation and structural features of the prepared compound was obtained by Raman spectroscopy. Raman analysis confirmed XRD results. Raman spectrum (Figure 2) of the sample displays the absorption bands typical for the various MeO6 (Me=V, Mo, Li) octahedral units building the lattice [8,12,14]. SEM images of the material at different magnifications are shown on Figure 3. As it is seen from the pictures, the prepared LiVMoO6 consists of dense agglomerates formed from irregular shaped particles in sub-micronic particles size range. Because of the high‐temperature technique adopted for the synthesis of LiVMoO6, it possesses a higher degree of crystallinity but as well as a higher degree of agglomeration as compared with LiVMoO6 product obtained by soft‐mechanochemical synthesis previously reported by us [12]. Its particles size and morphology is also different from the nano‐scale spherical LiVMoO6 product obtained by soft-mechanochemical synthesis [12]. These results evidenced the influence of the synthesis methods on the structural and morphological features of the LiVMoO6.
Electrochemical characterization
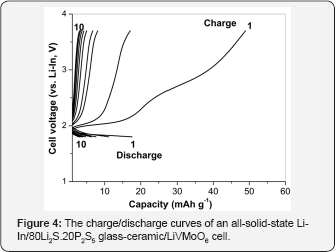
The electrochemical properties of all solid‐state‐cell using LiVMoO6 as an active material were examined. The cell was initially discharged (insertion of lithium ions into LiVMoO6 active material) and subsequently charged (extraction of lithium ions from LiVMoO6 active material) between 1.8 and 3.7 V under a current density of 0.1 mA cm‐2 at 25 °C. The discharge‐charge curves of the test cell for 10 cycles are shown in Figure 4. At the first discharge/charge cycle, the discharge capacity is about 18 mAh g‐1 and the charge capacity is about 50 mAh g‐1. The charge and discharge capacities began to fade immediately after the first cycle, dropping at about 83% by the 10th discharge. The poor electrochemical performance observed can be explained by the higher degree of aggregation of LiVMoO6 particles obtained by melt quenching resulting in worse contact with the solid electrolyte. As it is known, the favorable contact at electrode/ electrolyte solid interface is key to improve electrochemical performance of all‐solid-state batteries because charge‐transfer reaction proceeds only at the contact interface [15,16]. Another possible reason may be the structural degradation of LiVMoO6 during cycling [17]. The cycling behavior of the investigated all‐solid‐state cell differs from the cycling behavior of the previous reported all‐solid‐state cell where LiVMoO6 obtained by soft mechanochemical method was employed as a composite positive electrode [12]. Although LiVMoO6 synthesized by soft mechanochemical method also delivered a low specific capacity it showed a stable cycling behavior with a sustainable reversible capacity of 35 mAh g‐1 and a coulombic efficiency close to 100% after the second to the 10th cycles.
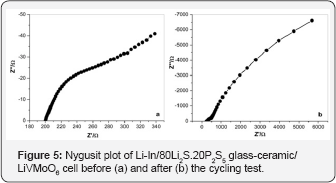
In order to understand the cycling behavior of the allsolid-state Li-In/LiVMoO6 cell, ac impedance measurements were carried out. Near capacitive response of both the fresh and cycled cells was detected characterized with incomplete semicircles with a large diameter in the shown Nyquist plots (Figure 5). Impedance results obtained also differ from the impedance results reported in reference [12], where the change in the blocking electrode behavior and an improvement of the electrochemical properties of the positive electrode upon cycling were observed
Conclusion
Single phase LiVMoO6, was successfully synthesized by applying of melt quenching method. Preparation of LiVMoO6 using high-temperature method of synthesis provides submicron-sized particles with irregular shape which are highly agglomerated. The electrochemical performance of the obtained LiVMoO6 as composite positive electrode in all-solid- state batteries was investigated. The battery shows unsatisfied electrochemical performance. A significant capacity fade occurs after 10 cycles at room temperature. It was suggested that the high degree of aggregation of LiVMoO6 particles resulting in worse contact with the solid electrolyte can be the main reason for the poor electrochemical results. The present result evidenced the importance of the synthesis method for the electrode properties.
Acknowledgment
Some of this work was done while the author M. Milanova was visiting the Department of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University under financial support by The Matsumae International Foundation (MIF) in the framework of the Matsumae International Fellowship Program April-September 2014. The same author wishes to thank all the members of Prof. Tatsumisago's group for their cooperation and support during her stay in Osaka Prefecture University.
Only you can see this message
This story is eligible to be part of the metered paywall. Learn more
Review of Green Geosynthetics by Using Biodegradable Resins for Civil Engineering Application as Tailor-Made Materials

Abstract
In this study, concept of green Geosynthetics was introduced in terms of biodegradability. Development of green Geosynthetics, its background and technical concerns were discussed through some research results of PLA (poly lactic acid) specimens. Test method for biodegradability of PLA (poly lactic acid) as a green Geosynthetics were considered and suggested based on composting method. Finally, the rest result shows that the concept of biodegradability for green Geosynthetics is available in the environmental application.
Keywords: Green geosynthetics; Biodegradability; Poly lactic acid; Environmental application
Go to
introduction
For eco-friendly environmental concept in Geosynthetics application fields, “Green” revolution is rapidly increasing in every construction sites in the world e.g., green structure, green installation, green industry etc. especially between construction and society’s needs. Furthermore, although durability of Geosynthetics should be emphasized for longterm service period, durability controlled mechanism could be required to fulfil the short-term degradability purpose for green Geosynthetics [1,2]. “Green Geosynthetics” can be defined as following: green Geosynthetics are made of eco-environmental biodegradable polymeric resins or natural materials and they must maintain their needed performance such as durability, design strength, hydraulic property etc. during service period in the application field. Then, after service period they should be degraded no harmful state in the soil structures [3,4]. In this article, environmental performance of green Geosynthetics was evaluated and reviewed to be related to the quantitative analysis of biodegradability of green Geosynthetics by conceptual consideration through its evaluation.
Experimental
Sample preparation: Three types of PLA (PLA 4032D, PLA 6201D; Nature works) and PBAT (poly (butylene adipate-co- terephthalate); BASF) were used as additives for performance improvement. Esterase and Phosphate buffered saline powder of pH 7.4 (Aldrich Co.) was used to investigate the degradation behavior. In this study, we used PLA 4032D resin for bleding with PBAT and PLA 6201D was used as reference material for comparison with PLA 4032D. Resins were vacuum dried at 60° for 4hrs before usage. Blending was performed with a Brabender Plastic order. Film specimens of PLA and PLA/PBAT blends were prepared through hot pressing under the pressure of 40kg/cm2 at 190°
Performance measurements
Physical properties: The molecular distribution of PLA resins was investigated with gel permeation chromatography (Waters GPC system, 515 pump, 2410 RI detector, Styragel column, PS standard). Tensile properties were evaluated with universal testing machine (Hounsfield, H1000KS).
Hydrolysis properties: To investigate the degradation behavior, strength retention was measured with PLA 4032D and 6201D. Degradation behavior in 0.01M phosphate buffered saline solution of pH 7.4 was monitored by incubation in a shaking water bath at 45.0±0.5 °C for up to 10 weeks. Tensile strength of incubated specimens was measured every 2 weeks using a tensile tester.
Enzymatic degradation: 63.6mg/150ml enzyme solution for bio-degradable resistance was made by 17 unit/mg of Esterase contained enzyme solution (Aldrich Co.) in pH 8.0 phosphate buffered saline solution. PLA film specimen was immersed in this solution for 4 weeks and strength retention was determined using the above equation.
Go to
Result and Discussion
Physical properties of PLA
As shown in (Table 1), PLA 6201D and 4032D showed similar poly dispersity index (PDI), but PLA 4032D had a slightly higher molecular weight.

Mechanical properties of PLA blend


In (Figure 1), tensile strength of PLA 4032D/PBAT blends decreased with increase of PBAT content. Especially, strength retention over PBAT content 40 wt% was less than that of 100% PBAT and this is due to compatibility decrease between PLA 4032D and PBAT by compounding. From this, it is seen that brittleness of PLA 4032D could be improved by blending with PBAT. Figure 2 shows the breaking strength of PLA 4032D with exposure temperature. In here, PLA 4032D blends were made to add PBAT which is a kind of biodegradable resin to improve flexibility of green Geosynthetics and strength decrease tendency is seen with PBAT blend ratio and temperature. From the slope of strength decay is very important because degradability control mechanism is determined through the half-life of strength analysis.
Environmental properties of PLA blend
Figure 3 shows the PLA 4032D specimen burial in soil and this shows strength retention of PLA 4032D under exposure condition and especially under activated sludge burial condition we can find the very rapid strength decay within 30 days. However, PLA shows almost 50% strength retention in soil burial condition within one year and this means green Geosynthetics of PLA can be available for one year if the strength decay slope could be controlled. To control biodegradability of PLA used green Geosynthetics, more restricted design technology must be adopted in the quality control and assurance of manufacturing and construction procedure in the installation field. Also, Figure 3 shows tensile strength of PLA 4032D specimen which is blended with PBAT content and it is seen that tensile strength decreased with PBAT content. For blending case of 50/50 PLA 4032D/PBAT, tensile strength decreased about 30% of 100 PLA 4032D used. This means the additive content is a kind of important factor to affect and control the bio-degradability of green Geosynthetics. Table 2 shows interface frictional coefficient between PLA specimen and soil by direct shear test for environmental application as Geosynthetics. In here, PLA 4032D/PBAT (80/20) shows improvement of interface frictional performance than PLA 4032D only used and this is an example of performance improvement by PBAT blending.


Proposal of biodegradability evaluation


Figure 4 shows the quantitative concept of biodegradability evaluation of green Geosynthetics and the best evaluation items should be selected in accordance with influence parameters which determine the long-term performance under real field installation conditions. In here, we can suggest a kind of hydrolysis method procedure of Figure 5 and this shows the evaluation procedure of degradability of PLA. ASTM D5338–98 (Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials under Controlled Composting Conditions) is introduced to simulate the real installation condition. Through the experimental data analysis, we can suggest the degradability test method with temperature as shown in here. Figure 6 by using Arrhenius plot of accelerated experimental data, we can predict the long-term biodegradable behaviors with temperature and induce this to designing the green Geosynthetics [5].
Go to
Conclusion
Through the overall environmental performance analysis of biodegradability as green Geosynthetics, it is seen that biodegradable mechanism of is possible to control theoretically and to control bio-degradability of PLA used green Geosynthetics. PLA 4032D/PBAT (80/20) blend shows improvement of environmental performance as a green Geosynthetics application than PLA 4032D only used. However, more restricted design technology must be adopted for this and more specific composition and selection of optimum additives of PLA blending should be determined for the quality control of PLA related Geosynthetics. To evaluate the biodegradability of green Geosynthetics performance, new test methods should be introduced and the needed evaluation items should be selected by considering influence parameters on the long-term performance under real field installation conditions.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
for more details click on the juniper publishers material science











Comments
Post a Comment